Australian universities are routinely causing traumatic brain injury (TBI) in rats and mice by dropping weights, or via invasive procedures to create fluid in the brain, in an attempt to replicate the human condition.
TBI animal experiments are carried out at University of Western Australia, Monash University, the University of Technology Sydney, University of Adelaide and the University of Melbourne. We are also aware of Australian pharmaceutical companies that employ the TBI experiment, although the animal testing may be outsourced overseas. Other times it is in conjunction with Australian universities.
University of Western Australia
In one closed head experiment (1) TBI was delivered to female adult rats using a custom built weight-drop device (figure 1). The animal’s head was placed directly under the guide tube and a 250g weight was released from a height of 1m onto the impact location. After impact the animal’s body fell 15 cm onto foam. Analgesia was then administered but the animals returned to consciousness 5 minutes after the injury. TBI was delivered repeatedly at one day intervals.
The University has recently acknowledged that animals are often not an ideal model for scientific research but HRA holds the view that no animal model truly represents the human condition and therefore results are unreliable.
Funding was provided to Melinda Fitzgerald by way of a Career Fellowship Grant from the National Health & Medical Research Council (NHMRC) (APP 1087114).
Figures (taken from the publication 1 )
Since this publication, researchers at the University of Western Australia have continued to use rats in TBI weight drop experiments – see references at the foot of page.
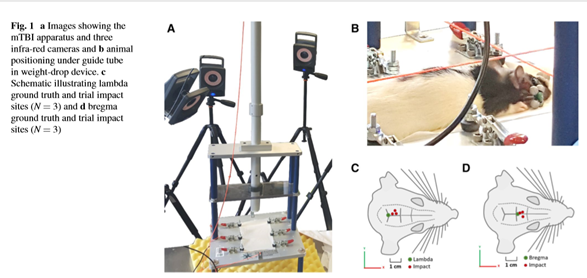
Credit: Repeated mild traumatic brain injury in female rats increases lipid peroxidation in neurons; Nathanael J. Yates · Stephen Lydiard · Brooke Fehily · Gillian Weir · Aaron Chin · Carole A. Bartlett · Jacqueline Alderson, and Melinda Fitzgerald. Exp Brain Res DOI 10.1007/s00221-017-4958-8 (Source – foot of page)
Monash University
Good News: As at October 2023 Monash University Animal Ethics Committee have advised HRA that “there are no current projects approved by our AEC that use TBI”.
Experimenters at Monash University use the weight drop acceleration method on male Sprague-Dawley rats.
In these experiments, a metal disk is fixed to the exposed skull using dental acrylic. The animal is then disconnected from a ventilator and placed under a ‘trauma device’ whilst a 450g weight is dropped from 2 metres through a vertical tube onto the metal disk in the brain (2). After injury the rats were then re-attached to the ventilator and breathing resumed after approximately 5 minutes. They were then taken off the ventilator and the metal disk was removed. Electrodes were then inserted into the brain for recording purposes.
This research (2) was to study progesterone as a neuroprotective treatment option although the researchers themselves state there is already “a wealth of preclinical data concerning progesterone treatment of TBI”.
This research was funded by the National Health and Medical Research (via grant APP 1029311 to Ramesh Rajan et al).
What is the Accelerated Weight Drop Injury?
For a better understanding as how researchers induce TBI in the rat, the video below shows footage of a rat (both illustration and footage) undergoing preparation for closed heat repeated weight drop injury (3)
University of Melbourne / Florey Institute
In experiments approved by the Florey Institute of Neuroscience and Mental Health, the University of Melbourne’s researchers have inflicted multiple traumatic brain injuries on the brains of mice both at 35 days of age and 70 days of age (4). After the TBI is caused, by way of blunt head trauma under a weight drop device, the mice undergo behavioural tests (including forced to swim for periods of time) to check for amnesia and gauge the severity of damage. The purpose was to study two development periods in the life of a mouse and test the hypothesis that a mild tBI during adolescence would predispose toward poor neurobehavioural and neuropathological outcomes after a subsequent injury at adulthood. In these mouse studies it did not – but we cannot rely on that for humans!
Again, the researchers were supported by grants from the NHMRC (being taxpayer dollars).
“Treatments that were neuroprotective in animals have, thus far, largely failed to translate in human clinical studies”
Dr Ashwin Kumaria, Department of Neurosurgery, Queen’s Medical Centre, Nottingham UK
In 2020, research from the University of Melbourne was published detailing research to develop a novel rat model that combines TBI, femoral fracture, and muscle crush injury (5). It involved a ‘novel’ rat model that combines TBI (via fluid to brain), muscle crush injury (a weight-drop apparatus was used to induce the muscle crush to the right hamstring. The hindlimb was positioned underneath a 1.2 kg impactor which was released from a height of 55cm. Immediately after the muscle injury the femoral fracture was performed by way of an incision to the patella, a small burrhole drilled into the femoral condyles using a 1.2 mm burr and wire inserted up the medullary cavity.
University of Adelaide
Experimenters at the University of Adelaide subjected rats to both moderate and repeated concussive traumatic brain injury(8). The rats were administered a substance and immunoreactivity in the cortex studied 24 hours after injury. Repeated injuries were delivered by a 400g weight drop onto the brain, 3 injuries at 5 days apart. The experiment was funded by the National Health and Medical Research Council.
University of Technology Sydney
The Animal Ethics Committee at the University of Technology Sydney approved experiments that subjected female Sprague-Dawley rats to the impact of a weight drop to their brains to cause injury (6) Immediately after the induction of the brain injury, the rats were administered antioxidant drugs that are already available (L-carnitine and Exendin-4). Neurological function was examined (24h and 6 weeks post injury) by way of, amongst other things, anxiety and motor function tests to assess cognition.
The antioxidants they used are existing medications, so why could the research not have been undertaken in ethically approved human trials of those with TBI?
Better Methods - In Vitro models
In his publication ‘In Vitro models as a platform to investigate traumatic brain injury’, Dr Ashwin Kumaria, Department of Neurosurgery at Queen’s Medical Centre, Nottingham, UK states that “Experimental in vivo models offer the potential to study TBI in the laboratory, however, treatments that were neuroprotective in animals have, thus far, largely failed to translate in human clinical studies. “In vitro (non animal) models of neurotrauma can be used to study specific pathophysiological cascades and to test potential neuroprotective strategies. These in vitro models include transection, compression, barotrauma, acceleration, hydrodynamic, chemical injury and cell-stretch methodologies. Various cell culture systems can also be utilized, including brain-on-a-chip, immortalized cell lines, primary cultures, acute preparations and organotypic cultures.
Examples of the potential of human in vitro and mathematical models is described in the following recent research publications.
Frankini E, Basile E J, Syed F, et al. (October 13, 2023) Understanding Traumatic Brain Injuries in Military Personnel: Investigating the Dynamic Interplay of the Cerebrospinal Fluid and Brain During Blasts. Cureus 15(10): e46962. doi:10.7759/cureus.46962
Biofidelic dynamic compression of human cortical spheroids reproduces neurotrauma phenotypes (2021)
Recent advancements in in vitro models of traumatic brain injury (2021)
Magnetoencephaloraphy (MEG)
Australia’s National Imaging Facility Annual Conference (Melbourne, 2023) held workshops showcasing new imaging modalities that can be used to improve human-relevant research in conditions affecting the brain such as epilepsy, autism, and traumatic brain injury (TBI). One such imaging modality is magnetoencephalography (MEG) which is promoted by the International Society for the Advancement of Clinical Magnetoencephalography (ISACM).
Published articles investigating the clinical use of MEG for TBI include A pilot treatment study for mild traumatic brain injury: Neuroimaging changes detected by MEG after low-intensity pulse-based transcranial electrical stimulation. (2017)
MEG can potentially improve the diagnosis for TBI if implemented and this has been recognised by the UK Ministry of Defence National Consensus Blast report: TBI Summit Post-Meeting Consensus Report 2020
Human Relevant Research
A broad range of human relevant research projects are being conducted at the University of Sydney’s Acquired Brain Injury Communication Lab. These projects are designed to improve the communication and life outcomes of people with acquired brain injury. Lead by Professor Leanne Togher, the Lab works with research participants with traumatic brain injury, aphasia following stroke, dementia and other acquired neurological communication disorders.
The National Imaging Facility, partnering with AUS-mTBI is a national consortium working to build Australia’s first clinical and imaging data resource of people experiencing mTBI. The MRI technology will improve understanding of each person’s brain injury biology.
An example of human relevant research can be found here at this publication. Mechanical Stretching-Induced Traumatic Brain Injury Is Mediated by the Formation of GSK-3β-Tau Complex to Impair Insulin Signaling Transduction
CELLINK has facilitated a multicellular 3D model to represent the intricacies of the brain’s 3D microvasculature and the microfluidic properties of in vivo vessels with the light-based LUMEN X™ 3D bioprinter. Read more here.
Relevance to Humans
According to Dr Andre Menache, BSc (Zoology), BSc(Hons), BVSc, MRCVS, “The study by these researchers falls under the heading of basic research, which by definition, makes no claim to clinical application. Animal researchers conducting these basic studies typically make some reference to human conditions in order to justify the funding of such endeavours”.
“The choice of the rat to study traumatic brain injury ignores species differences and evolutionary biology. The rat possesses a four layered lissencephalic cortex (humans possess a six layered gyrencephalic cortex) and are separated by 70 million years of evolution. These facts have important consequences when trying to extrapolate results from animal studies to humans. As an example, more than 1000 drugs have demonstrated neuroprotection in animal models (mostly rats) of experimental stroke. However, none have shown neuroprotection in humans. This represents a success rate of zero.”
Dr André Ménache, BSc(Hons) BVSc MRCVS, Director of Antidote Europe,
“Animal models of TBI incompletely represent the human situation in a number of ways, particularly with regard to size considerations and extensive anatomical and histological differences”
Dr Asin Kumaria, Department of Neurosurgery, Queen’s Medical Centre, Nottingham UK
Cost Benefit Analysis
The Australian and New Zealand Council for the Care of Animals in Research and Teaching states that: “Using animals for scientific purposes is acceptable only when any harm done to the animals is very greatly outweighed by the benefits of their use”. These studies are clearly an example of basic research which is defined as “experimental or theoretical work undertaken primarily to acquire new knowledge of the underlying foundations of phenomena and observable facts, without any particular application or use in view” (OECD definition).”
It is unclear in each case as to whether the authors could foresee any application of this work. The publications do not provide any evidence to show that the information obtained from the killing of these animals will lead to benefits for people with traumatic brain injury.
Studies of sensory processes and deficits in humans suffering TBI should instead be relied upon to provide information regarding cognitive processing and possible rehabilitation.
All three research projects described here received government funding of one type or another – either by way of a substantial grant or for Career Development from the National Health and Medical Research Council (NHMRC) thereby Australian taxpayer funded.
What can you do?
Please use the form below to tell the National Health and Medical Research Council (NHMRC) how disappointed you are that the NHMRC funds animal research such as these traumatic brain injury experiments in rats and mice. You can use the text provided or compose your own.
Your message will be sent via email to the NHMRC.
"*" indicates required fields
You can also send your objections to the following institutions:
Monash University
Prof Margaret Gardner
Vice Chancellor,
Monash University Vic 3800
Margaret.gardner@monash.edu.au
University of WA
Prof Amit Chakma
Vice Chancellor
The University of Western Australia
35 Stirling Highway
PERTH WA 6009
Vice-chancellor@uwa.edu.au
Uni. of Melbourne
Prof Duncan Maskell
Vice Chancellor
University of Melbourne
PARKVILLE Vic 3010
vc@unimelb.edu.au
UTS Sydney
Professor Andrew Parfitt
Vice-Chancellor and President
UTS, 15 Broadway, Ultimo NSW 2007
Email via EA samantha.sandford@uts.edu.au
University of Adelaide
Professor Peter Høj
Vice Chancellor President
Email: vice-chancellor@adelaide.edu.au
References
- Repeated mild traumatic brain injury in female rats increases lipid peroxidation in neurons; Nathanael J. Yates · Stephen Lydiard · Brooke Fehily · Gillian Weir · Aaron Chin · Carole A. Bartlett · Jacqueline Alderson, and Melinda Fitzgerald
Exp Brain Res (2017) DOI 10.1007/s00221-017-4958-8 (Source: Repeated mild traumatic brain injury in female rats increases lipid peroxidation in neurons – UQ eSpace) - Progesterone Sharpens Temporal Response Profiles of Sensory Cortical Neurons in Animals Exposed to Traumatic Brain Injury
Benjamin J. Allitt, Victoria P. A. Johnstone, Katrina L. Richards, Edwin B. Yan, and Ramesh Rajan
Cell Transplantation 2017, Vol. 26(7) 1202-1223 - A Novel Model of Mild Traumatic Brain Injury for Juvenile Rats.
Mychasiuk, R., Farran, A., Angoa-Perez, M., Briggs, D., Kuhn, D., Esser, M. J.; J. Vis. Exp. (94), e51820, doi:10.3791/51820 (2014) - Mild Traumatic Brain injury in adolescent Mice alters skull Bone Properties to influence a subsequent Brain impact at adulthood: a Pilot study
Thomas J. McColl, Rhys D. Brady, Sandy R. Shultz, Lauren Lovick, Kyria M. Webster, Mujun Sun, Stuart J. McDonald, Terence J. O’Brien and Bridgette D. Semple
Frontiers in Neurology 2018, Vol 9 Article 372 - A novel rat model of heterotopic ossification after polytrauma with traumatic brain injury.Brady RD, Zhao MZ, Wong KR, et al. Bone. 2020;133:115263. doi:10.1016/j.bone.2020.115263
- L-Carnitine and extendin-4 improve outcomes following moderate brain contusion injury. Chen, H., Chan, Y.L., Linnane, C. et al. . Sci Rep 8, 11201 (2018). https://doi.org/10.1038/s41598-018-29430-6
- Further University of Western Australia barbaric TBI weight drop experiments include the following publications – Frontiers | Repeated Long-Term Sub-concussion Impacts Induce Motor Dysfunction in Rats: A Potential Rodent Model | Neurology (frontiersin.org); Differential responses to increasing numbers of mild traumatic brain injury in a rodent closed head injury model (curtin.edu.au); The effects of a combination of ion channel inhibitors in female rats following repeated mild traumatic brain injury (curtin.edu.au) and Elevation of oxidative stress indicators in a pilot study of plasma following traumatic brain injury (curtin.edu.au)
NK1 antagonists attenuate tau phosphorylation after blast and repeated concussive injury Corrigan, F., Cernak,I., McAteer, K., Hellewell, S.C., Rosenfeld, JV, Turner, R.J & Vink,R Scientific Reports Vol 11, Article number: 8861 (2021) o
Featured image by Nikolett Emmert on Unsplash
